For US Healthcare Professionals
XARELTO®: Efficacy profile for prophylaxis of venous thromboembolism (VTE) in acutely ill medical patients◇
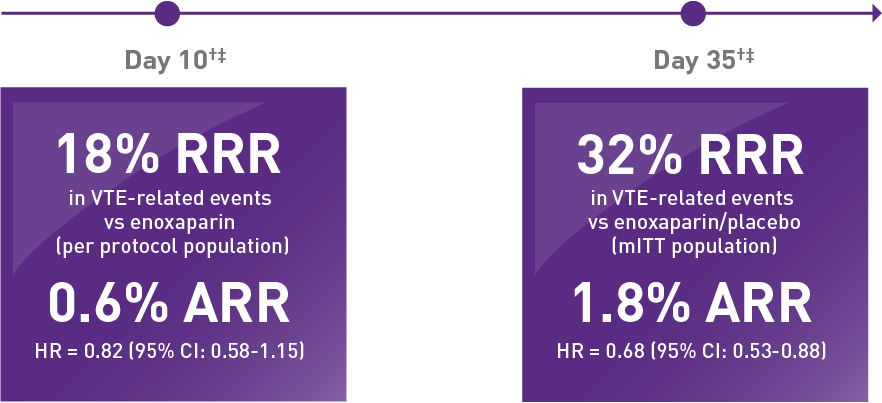
VTE RELATIVE RISK REDUCTION IN THE MAGELLAN SUBGROUP
EFFICACY RESULTS AT DAY 10 AND DAY 35 IN ACUTELY ILL MEDICAL PATIENTS VERSUS STANDARD OF CARE IN THE MAGELLAN SUBGROUP◇*1,2
-
At Day 10 and Day 35, XARELTO® demonstrated lower rates of VTE when compared with enoxaparin/placebo
- Day 10: 2.4% (58/2385) of patients taking XARELTO® experienced a VTE event versus 3.0% (72/2433) of patients taking enoxaparin
- Day 35: 3.9% (94/2419) of patients taking XARELTO® experienced a VTE event versus 5.7% (143/2506) of patients taking enoxaparin/placebo
MAGELLAN1
Trial design: Randomized, multicenter, double-blind, parallel-group efficacy and safety study comparing XARELTO® with enoxaparin, in the prevention of VTE in hospitalized acutely ill medical patients during the in-hospital and post–hospital-discharge period. Eligible patients included adults who were at least 40 years of age, hospitalized for an acute medical illness, at risk of VTE due to moderate or severe immobility, and had additional risk factors for VTE. The population at risk of VTE was required to have 1 or more of the following VTE risk factors: prolonged immobilization, age ≥75 years, history of cancer, history of VTE, history of heart failure, thrombophilia, acute infectious disease contributing to the hospitalization, and BMI ≥35 kg/m2. The causes for hospitalization included heart failure, active cancer, acute ischemic stroke, acute infectious and inflammatory disease, and acute respiratory insufficiency. Patients were randomized to receive either XARELTO® 10 mg once daily for 35 ±4 days starting in hospital and continuing post hospital discharge (n=4050) or enoxaparin 40 mg once daily for 10 ±4 days starting in hospital followed by placebo post discharge (n=4051).
Primary outcomes: The primary efficacy outcome was a composite endpoint that included asymptomatic proximal DVT in lower extremity, symptomatic proximal or distal DVT in the lower extremity, symptomatic nonfatal PE, and death related to VTE.
◇For VTE prophylaxis in acutely ill medical patients at risk for thromboembolic complications who are not at high risk of bleeding.
*Patients excluded due to high risk of bleeding: history of bronchiectasis, pulmonary cavitation, or pulmonary hemorrhage; active cancer (ie, undergoing acute, in-hospital cancer treatment); active gastroduodenal ulcer in the 3 months prior to treatment; history of bleeding within the last 3 months prior to treatment; or receiving DAPT.
†Primary efficacy outcomes: the composite of asymptomatic proximal DVT, symptomatic proximal or distal DVT, symptomatic nonfatal PE, or VTE-related death.
‡Patients received either XARELTO® or placebo once daily for 35 days ±4 days starting in hospital and continuing post hospital discharge, or received enoxaparin or placebo once daily for 10 days ±4 days in the hospital.
§The decision regarding initiation setting should be based on the prescriber's clinical judgment.
ARR = absolute risk reduction; BMI = body mass index; DAPT = dual antiplatelet therapy; DVT = deep vein thrombosis; mITT = modified intent-to-treat; PE = pulmonary embolism; RRR = relative risk reduction.

