For US Healthcare Professionals
XARELTO® vascular dose*: The FIRST and ONLY treatment approach indicated to help reduce risk† in patients with PAD post LER1,2
COMPARABLE INCIDENCE IN PRINCIPAL SAFETY OUTCOME VERSUS PLACEBO‡§1,2
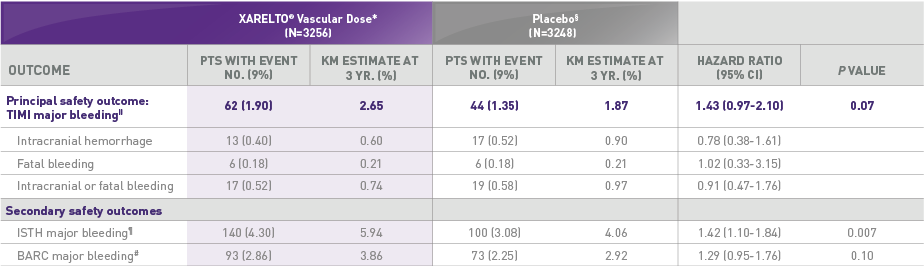
Principal safety outcome:
- No significant difference in TIMI major bleeding between the XARELTO® vascular dose* and placebo§ was observed
TIMI major bleeding = fatal bleeding, intracranial hemorrhage, a decrease in hemoglobin level of ≥5 g/dL, or a decrease in hematocrit of ≥15%.
ISTH major bleeding = fatal bleeding, bleeding into a critical site, a decrease in hemoglobin level of ≥2 g/dL, or transfusion of at least 2 units of packed red cells or whole blood.
BARC = major bleeding is defined as grade 3b or higher.
In a prespecified subgroup analysis of the VOYAGER trial, patients were administered clopidogrel at the investigator's discretion**3:
ABSOLUTE RISK REDUCTION† CONSISTENT WITH ADDITION OF XARELTO® VASCULAR DOSE* REGARDLESS OF CLOPIDOGREL USE‡††
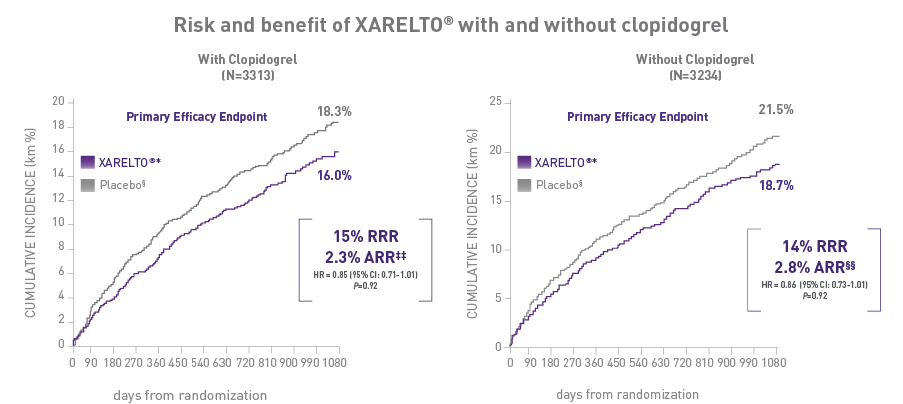
- The XARELTO® vascular dose* consistently affected the risk of adverse CV and limb events|| || with an early benefit for acute limb ischemia, regardless of clopidogrel use. Limb and CV risk reductions remained consistent over time
TIMI major bleeding similar to placebo
-
With concomitant clopidogrel:
1.94% on XARELTO® and 1.47% on placebo§¶¶:
HR = 1.33 (95% CI: 0.78-2.26), P=0.71; ARI = 0.5% -
Without concomitant clopidogrel:
1.87% on XARELTO® and 1.25% on placebo§##:
HR = 1.55 (95% CI: 0.88-2.72), P=0.71; ARI = 0.6%
Increase in ISTH major bleeding
-
The addition of clopidogrel, for the duration of >30 days, caused an increase in ISTH major bleeding:
HR = 3.20 (95% CI: 1.44-7.13)
The XARELTO® vascular dose*
PROVEN SAFETY PROFILE FOR PATIENTS WITH CAD AND/OR PAD1
|
XARELTO® vascular dose* (2.5 mg BID with aspirin 100 mg QD) |
Placebo + aspirin (100 mg QD) |
|---|---|
| Event rate (%/year [n/N]) | |
|
Major bleeding*** HR (95% CI): 1.8 (1.5, 2.3) |
|
| 1.6%(263/9134) | 0.9%(144/9107) |
|
Fatal bleeding event HR (95% CI): 1.5 (0.6, 3.7) |
|
| <0.1%(12/9134) | <0.1%(8/9107) |
|
Symptomatic bleeding in critical organ (nonfatal) HR (95% CI): 1.4 (0.9, 2.0) |
|
| 0.3% (58/9134) | 0.3%(43/9107) |
|
Bleeding into surgical site requiring reoperation (nonfatal, not in critical organ) HR (95% CI): 1.2 (0.4, 3.5) |
|
| <0.1%(7/9134) | <0.1%(6/9107) |
|
Bleeding leading to hospitalization (nonfatal, not in critical organ, not requiring reoperation) HR (95% CI): 2.1 (1.6, 2.7) |
|
| 1.1%(188/9134) | 0.5%(91/9107) |
- Major bleeding was increased; however, ~97% of patients taking the XARELTO® vascular dose* did not experience major bleeding1
The incidences of major bleeding*** in the CAD and PAD populations in COMPASS were comparable.1
ARI = absolute risk increase; ARR = absolute risk reduction; BARC = Bleeding Academic Research Consortium; BID = twice daily; CAD = coronary artery disease; CV = cardiovascular; CVD = cardiovascular disease; ISTH = International Society on Thrombosis and Haemostasis; KM = Kaplan-Meier; LER = lower extremity revascularization; PAD = peripheral artery disease; PTS = patients; QD = once daily; RRR = relative risk reduction; TIMI = Thrombolysis in Myocardial Infarction.
*XARELTO® 2.5 mg twice daily plus aspirin (100 mg in clinical trial; 75 mg to 100 mg in practice) once daily.
†Reduction of a composite of myocardial infarction, ischemic stroke, acute limb ischemia, and major amputation of a vascular etiology in patients with PAD, including patients who have recently undergone an LER procedure due to symptomatic PAD.
‡Treatment schedule: XARELTO® 2.5 mg twice daily versus placebo; all patients received aspirin 100 mg once daily as background therapy.
§Patients on placebo received aspirin 100 mg once daily.
IITIMI major bleeding is defined as fatal bleeding, intracranial hemorrhage, a decrease in hemoglobin level of ≥5 g/dL, or a decrease in hematocrit of ≥15%.
¶ISTH major bleeding is defined as fatal bleeding, bleeding into a critical site, a decrease in hemoglobin level of ≥2 g/dL, or transfusion of at least 2 units of packed red cells or whole blood.
#BARC major bleeding is defined as grade 3b or higher.
**Clopidogrel use was not a randomized therapy and was associated with a different patient profile of CV risk, bleeding risk, and burden of concomitant CVD between the groups; direct comparisons of the efficacy and safety of clopidogrel in this setting are confounded, particularly without adjustment.
††Clopidogrel could be administered for up to 6 months.
‡‡ARR = 18.3%-16.0% = 2.3%.
§§ARR = 21.5%-18.7% = 2.8%.
|| ||Composite endpoint: acute limb ischemia, major amputation for a vascular cause, myocardial infarction, ischemic stroke, or CV death.
¶¶ARI = 1.94%-1.47% = 0.5%.
##ARI = 1.87%-1.25% = 0.6%.
***Major bleeding events within each subcategory were counted once per patient, but patients may have contributed events to multiple subcategories. These events occurred during treatment or within 2 days of stopping treatment in the safety analysis set.

