For US Healthcare Professionals
For US Healthcare Professionals
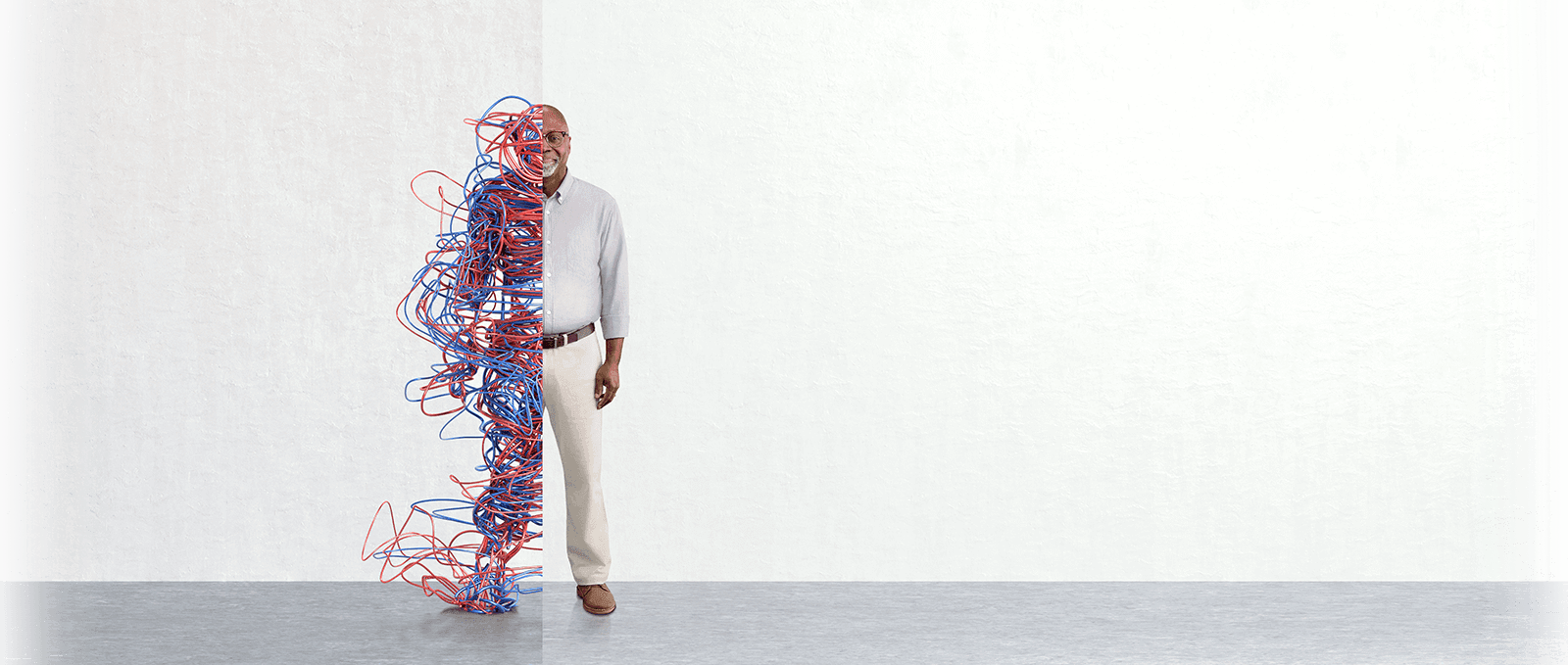
Helping protect them against thrombosis doesn’t have to be.
Intentionally developed to help protect against thrombotic events, providing unique versatility across the broadest range of patients in its class
ACCESS AND AFFORDABILITY Help your patients start and stay on therapy
Choose Savings/Support —
EFFICACY Proven efficacy profile across 9 adult indications
Choose Indication —
SAFETY Proven safety profile across 9 adult indications
Choose Indication —
CAD = coronary artery disease; CV = cardiovascular; DVT = deep vein thrombosis; NVAF = nonvalvular atrial fibrillation; PAD = peripheral artery disease; PE = pulmonary embolism; RCTs = randomized clinical trials; VTE = venous thromboembolism.