For US Healthcare Professionals
XARELTO®: Clinical trial for venous thromboembolism (VTE) prophylaxis in acutely ill medical patients◇
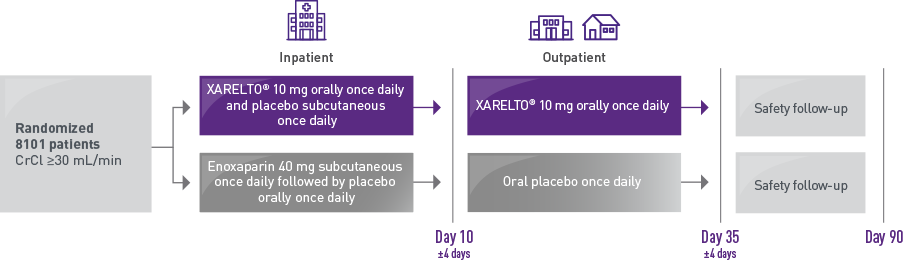
The MAGELLAN trial studied acutely ill medical patients at risk for a VTE in both inpatient and outpatient settings1
Based on other Analyses, 5 additional exclusion criteria were applied to create the MAGELLAN subgroup1

Who were the patients evaluated for inpatient and extended VTE prophylaxis in the MAGELLAN subgroup?1,2

Exclusion Criteria
Five key risk factors for major bleeding were identified and applied as exclusion criteria to MAGELLAN overall:
- History of bronchiectasis, pulmonary cavitation, or pulmonary hemorrhage.
- Active cancer (ie, undergoing acute, in-hospital cancer treatment).
- Active gastroduodenal ulcer in the 3 months prior to treatment.
- History of bleeding within the last 3 months prior to treatment.
- Receiving DAPT.
◇For VTE prophylaxis in acutely ill medical patients at risk for thromboembolic complications who are not at high risk of bleeding.
*Major bleeding was defined as clinically overt bleeding associated with a drop in hemoglobin of ≥2 g/dL, a transfusion of ≥2 units of packed red blood cells or whole blood, bleeding at a critical site, or with a fatal outcome.
†In addition to the aforementioned, additional VTE risk factors include D-dimer levels that are elevated beyond 2 times the upper limit of normal, chronic venous insufficiency, severe varicosis, recent major surgery, and recent serious trauma (6 to 12 weeks).3
‡The decision regarding initiation setting should be based on the prescriber's clinical judgment.
BMI = body mass index; CrCl = creatinine clearance; DAPT = dual antiplatelet therapy; PE = pulmonary embolism; VTE = venous thromboembolism.