COMPASS trial
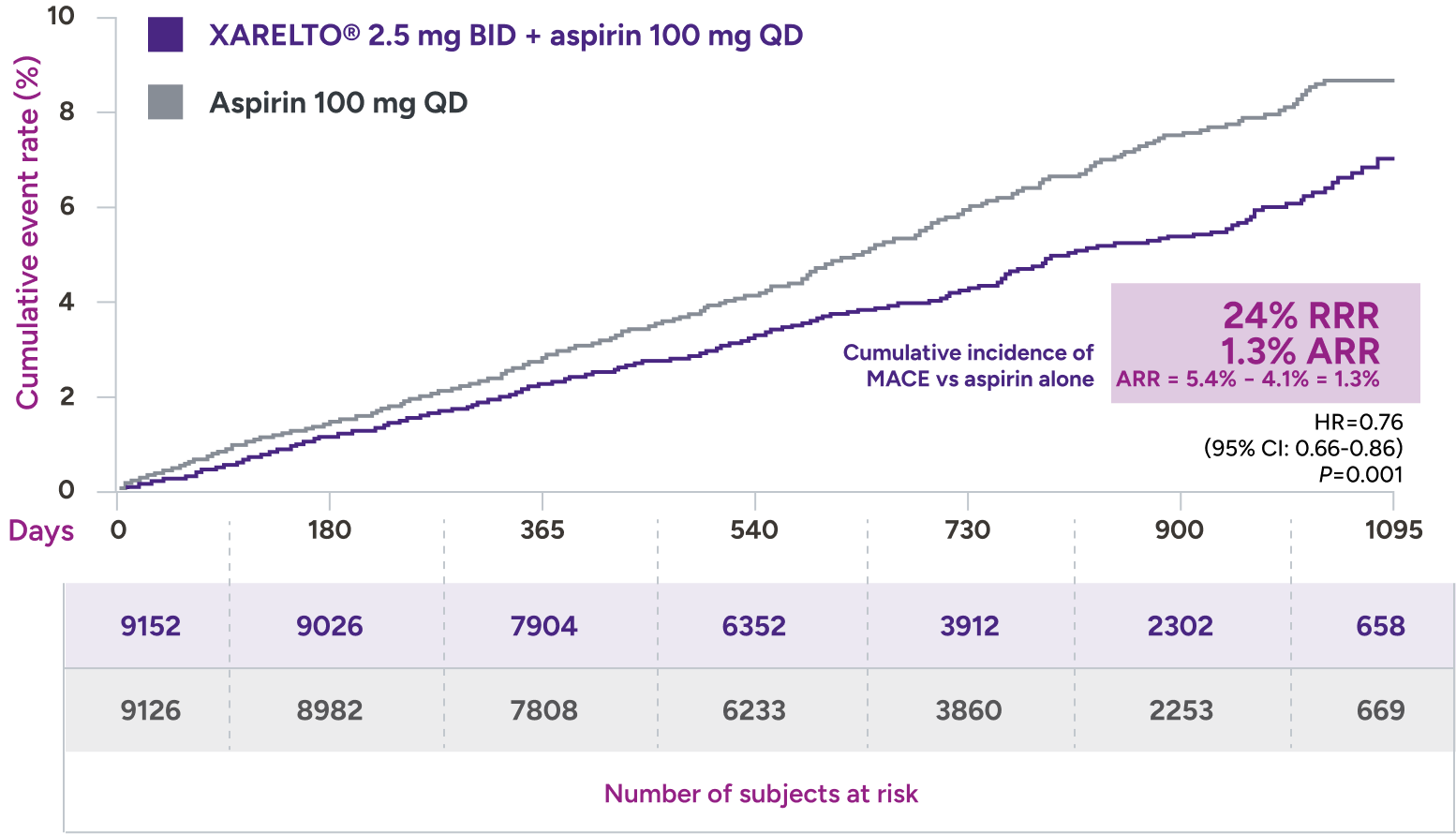
XARELTO® 2.5 mg with aspirin* significantly reduced a composite of CV death, MI, and stroke in patients with CAD/PAD5,6
*XARELTO® 2.5 mg twice daily plus aspirin 100 mg once daily.
Primary outcome: major cardiovascular events†
all-cause mortality‡
HR=0.82 (95% CI: 0.71-0.96)
+ aspirin 100 mg QD:
3.4% (313/9152)
vs
Aspirin 100 mg QD:
4.1% (378/9126)
QD = once daily; RRR = relative risk reduction.
COMPASS trial
| Clinical endpoint | XARELTO® 2.5 mg BID + aspirin 100 mg QD %/year | (n/N) | Placebo (aspirin 100 mg QD) %/year | (n/N) | HR (95% CI) | Notes |
| Major bleeding* | 1.6 (263/9134) | 0.9 (144/9107) | 1.8 (1.5, 2.3) | |
| Fatal bleeding event | <0.1 (12/9134) | <0.1 (8/9107) | 1.5 (0.6, 3.7) | |
| Symptomatic bleeding in critical organ (nonfatal) | 0.3 (58/9134) | 0.3 (43/9107) | 1.4 (0.9, 2.0) | |
| Bleeding into surgical site requiring reoperation (nonfatal, not in critical organ) | <0.1 (7/9134) | <0.1 (6/9107) | 1.2 (0.4, 3.5) | |
| Bleeding leading to hospitalization (nonfatal, not in critical condition, not requiring reoperation) | 1.1 (188/9134) | 0.5 (91/9107) | 2.1 (1.6, 2.7) |
≤1%
of patients had fatal bleeding, nonfatal ICH, or critical organ bleeding1
*Major bleeding events within each subcategory were counted once per patient, but patients may have contributed events to multiple subcategories. These events occurred during treatment or within 2 days of stopping treatment in the safety analysis set.
BID = twice daily; CAD = coronary artery disease; CI = confidence interval; HR = hazard ratio; ICH = intracranial hemorrhage; ISTH = International Society on Thrombosis and Haemostasis; PAD = peripheral artery disease; QD = once daily.
Dosing
Reducing the risk of major CV events* in patients with CAD

2.5 mg
2.5 mg twice daily
With or without food, in combination with aspirin (75 mg-100 mg) once daily
No dose adjustment needed based on CrCl
Tablet shown not actual size.
*Major cardiovascular events were a composite of cardiovascular death, myocardial infarction, and stroke.
CAD = coronary artery disease; CrCl = creatinine clearance; CV = cardiovascular.

