For US Healthcare Professionals
Similar rates of the composite of clinically relevant nonmajor and major bleeding in EINSTEIN PE*1
10.3% (249/2412) with XARELTO® versus 11.4% (274/2405) with enoxaparin and warfarin/VKA HR (95% CI): 0.90 (0.76-1.07)
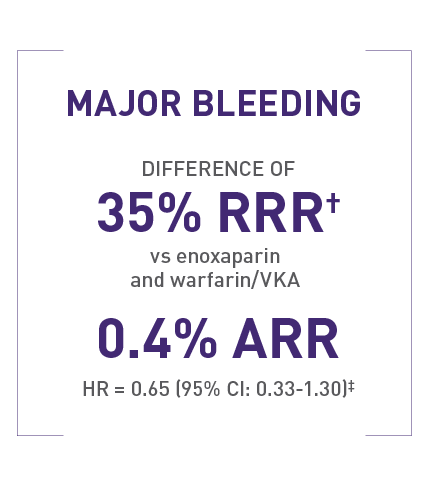
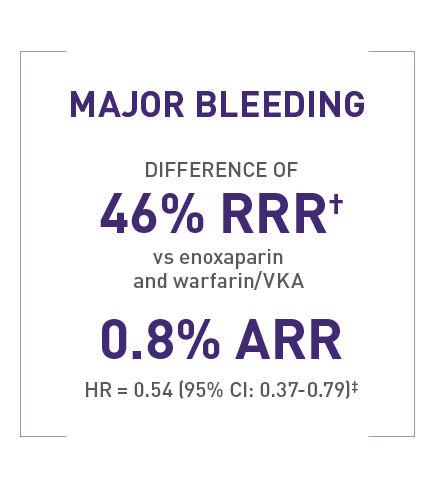
Rates of major bleeding in EINSTEIN PE1
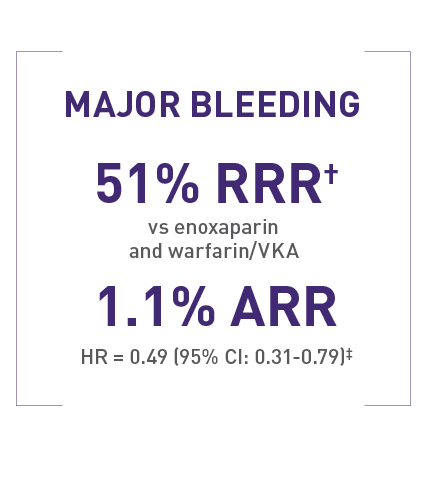
1.1% (26/2412) with XARELTO® versus 2.2% (52/2405) with enoxaparin and warfarin/VKA